Milestones in non-animal safety testing
With skin engineering

1979
1st RECONSTRUCTED HUMAN EPIDERMIS MODEL
For the 1st time worldwide, L’OREAL biologists were able to reconstruct a living epidermis, from human skin cell
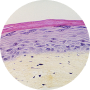
1986
1st RECONSTRUCTED HUMAN FULL SKIN MODEL
For the 1st time worldwide, L’OREAL biologists were able to reconstruct living skin with a living epidermis and a living dermis, from human skin cells
1989
L’OREAL STOPS TESTING ITS PRODUCTS ON ANIMALS
The European Regulation will not require this for another 14 years (2003)

1994
1st RECONSTRUCTED HUMAN PIGMENTED EPIDERMIS MODEL
For the 1st time worldwide, L’OREAL biologists were able to reconstruct a living epidermis, from human skin cells, that is able to tan.
1995
L’ORÉAL BIOLOGISTS RECEIVE THE PRIX AMALTHÉE FROM OPAL IN FRANCE
For the development and use of reconstructed skin.
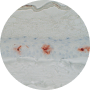
1997
1st RECONSTRUCTED HUMAN EPIDERMIS MODEL WITH LANGERHANS CELLS
For the 1st time worldwide, L’OREAL biologists were able to reconstruct a living epidermis, from human skin cells, that shows the key role played by these specialist cells
CREATION OF THE EPISKIN SUBSIDIARY IN LYON (FRANCE) EPISKIN,
the world leader in tissue engineering, offers human reconstructed tissues to the global scientific community (academic and industry) to support research and development activities in safety and efficacy.
1998
ECVAM VALIDATION OF THE EPISKIN MODEL TO ASSESS SKIN CORROSION
Official recognition of our Episkin model to assess skin corrosion.
1999
L’ORÉAL DECIDES TO ABANDON ALL INGREDIENTS FROM BOVINE ORIGIN
ECVAM VALIDATION OF THE EPISKIN MODEL TO ASSESS PERCUTANEOUS ADSORPTION
Official recognition of our Episkin model to assess whether ingredients can pass through the skin into the blood stream
2000
CONSTRUCTION OF THE EPISKIN CENTER IN LYON (GERLAND)

2001
ECVAM VALIDATION OF THE EPISKIN MODEL TO ASSESS CHEMICAL CORROSION
Official recognition of our Episkin model to assess the impact of chemicals on the skin
2002
OECD VALIDATION OF THE EPISKIN MODEL TO ASSESS PHOTOTOXICITY
Official recognition of our Episkin model to assess the impact of light (including UV) on skin.
2003
EUROPEAN TESTING BAN ON ANIMALS FOR COSMETIC PRODUCTS
Ban of marketing of finished cosmetic products tested on animals
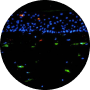
2006
EPISKIN ACQUIRES SKINETHIC COMPANY
Episkin, our subsidiary dedicated to the development of reconstructed skin models, acquires SkinEthic an expert in the field of tissue engineering to widen our expertise.
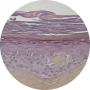
2007
ECVAM VALIDATION OF THE EPISKIN MODEL TO ASSESS SKIN IRRITATION
Official recognition of our Episkin model to assess skin irritation
2008
1st RECONSTRUCTED HUMAN PIGMENTED FULL SKIN MODEL
For the 1st time worldwide, L’OREAL biologists are able to reconstruct a living skin (epidermis + dermis), with human skin cells, that is able to tan.
2009
EUROPEAN MARKETING BAN ON ANIMAL TESTING
This ban prohibits to marketing finished cosmetic products and ingredients in the EU, which were tested on animals. But testing is still allowed for some complex health effects (cancer, allergens…).
2010
FIRST RECONSTRUCTED SKIN MODELS PRODUCED IN OUR CHINESE LABS IN SHANGHAI
OECD VALIDATION OF THE EPISKIN AND SKINETHIC MODELS TO ASSESS SKIN IRRITATION
Official recognition of our Episkin model to assess skin irritation.
2011
INAUGURATION IN LYON OF EPISKIN, THE 1st PREDICTIVE EVALUATION CENTER OF THE COSMETIC INDUSTRY
This center houses more than 75 biologists over a surface area of 3670 m² of laboratories -including 1000 m² of cleanrooms, where the reconstructed tissues are produced. Each year, the center produces approximately 100,000 units of reconstructed tissues, and its platforms evaluate thousands of formulas and a hundred-some ingredients.
2013
FULL EUROPEAN MARKETING BAN ON ANIMAL TESTING
Full ban enters into force. This is the end of animal suffering for cosmetic reasons.
2014
L’ORÉAL OBTAINS A BUSINESS LICENCE FOR RECONSTRUCTED SKIN MODELS EPISKIN IN CHINA
L'Oréal has been granted a Chinese business licence for Shanghai Episkin Biotechnology Ltd. The Chinese Episkin model is a human epidermis model reconstructed from Asian keratinocytes, produced by the L’Oréal laboratories in China according to the same strict production standard and quality control criteria as at L’Oréal’s Predictive Evaluation Center in Lyon, France
ANIMAL TESTING EVOLUTION IN CHINA
Some products manufactured and sold in China, such as shampoos, shower gels and some make-up products are no longer tested on animals.
2017
OECD VALIDATION OF 2 ALTERNATIVES TO ANIMAL TESTING, DEVELOPED BY L’ORÉAL, TO ASSESS SKIN ALLERGY AND EYE IRRITATION.
Official recognition of our alternative methods to assess skin allergy and eye irritation.
2019
OPENING OF THE EPISKIN CENTER IN RIO DE JANEIRO (BRAZIL)
With the launch of EPISKIN in Brazil, the reconstructed human skin models and all associated safety methods are available to the Brazilian and Latin American scientific community for product testing purposes.
2020
PARTNERSHIP WITH AFSA, SCIENTIFIC COLLABORATION PROGRAM OF HSI
L'Oréal becomes an active member of the AFSA executive committee after having been collaborating from a scientific point of view for several years.
Through scientific collaboration with the AFSA/HSI, we can work together to promote animal-free methods through training and education and share best practices widely.
2021
FURTHER ANIMAL TESTING POSITIVE EVOLUTION IN CHINA
Since 2014, some products manufactured and sold in China, such as shampoos, shower gels and some make-up products, are no longer tested on animals. In May 2021, these same products imported into China will no longer be tested on animals provided that they can show a certification of compliance with Good Manufacturing Practices from the country in which they are manufactured and a safety assessment.
GLOSSARY
Chemical corrosion: A gradual destruction of skin by chemicals
Epidermis: The outermost layer of the skin
Dermis: The innermost layer of the skin
ECVAM: European Center for Validation of Alternative Methods to animal testing
EURL-ECVAM : European Union Reference Laboratory for Alternatives to Animal Testing
Eye irritation: A reaction leading to redness or a burning sensation, which may or may not be reversible and that affects the cornea or the conjunctiva of the eye.
HSI : One of the most influential NGOs on worldwide regulatory changes in the area of alternative methods to animal testing.
Langerhans Cells : Cells linked to our immune system
OECD : Organisation for Economic Co-operation and Development
OPAL : Œuvre d’Assistance aux Animaux de Laboratoire
Percutaneous absorption : The passage of substances through the skin, thus reaching the blood stream.
Phototoxicity : Chemically induced skin irritation, requiring light, and that induces on the skin a response resembling to an exaggerated sunburn.
Skin Allergy : A delayed immunological reaction that occurs following repeated contact with a sensitizing substance from the environment, a chemical or a cosmetic product.
Skin Corrosion : The production of irreversible damage of the skin
Skin Irritation : A skin reaction caused by allergens, heat, or certain medical conditions, and can include skin bumps, scaly or red skin, and itchy or burning skin.